September 4, 2024
Disordered regions in the IRE1α ER lumenal domain mediate its stress-induced clustering
During periods of protein folding stress, the transmembrane protein IRE1 detects misfolded proteins in the endoplasmic reticulum (ER) and initiates a transcriptional relay as part of the unfolded protein response (UPR). In order to signal the presence of unfolded proteins in the ER, IRE1 oligomerizes, but the molecular mechanisms behind this process remain unknown. In a recent study from the Karagöz lab at the Max Perutz Labs, published in EMBO Journal, first authors Paulina Kettel, PhD student, and Laura Marosits, then research associate, provide the first evidence that disordered regions in IRE1’s ER luminal sensor domain regulate its self-assembly. Their discoveries could help pave the way for the development of drugs that target diseases associated with protein misfolding.
The ER is a critical organelle responsible for the folding of approximately 30% of the proteome. When misfolded proteins accumulate in the ER, the unfolded protein response (UPR) halts protein translation, targets the misfolded proteins for degradation, and activates transcription factors that drive the expression of chaperones to aid protein folding. If overloaded, the UPR triggers apoptosis. Group leader Elif Karagöz explains: “The UPR, crucial for protein homeostasis, is often hijacked in metabolic diseases or cancer, where a weaker UPR benefits tumor survival, making its study critically important.” Conversely, in neurodegenerative and metabolic diseases, UPR overactivation becomes harmful, and inhibiting it is a key treatment strategy.
Under normal conditions, the chaperone BiP keeps IRE1 inactive, but BiP inhibition of IRE1 is released upon the sensing of misfolded proteins. Misfolded proteins bind to IRE1’s ER lumenal domain and triggers IRE1 to self-assemble into clusters, bringing its cytosolic kinase and RNAse domains into close proximity, leading, ultimately, to the expression of chaperone genes. While IRE1 from multiple species has been observed to oligomerize, driven by its ER-luminal sensor domain, the precise mechanisms of its polymerization remain unclear.
The Karagöz lab found, unexpectedly, that intrinsically disordered regions (IDRs) within the luminal sensor domain of IRE1 are crucial for its regulation. First author and PhD student in the Karagöz lab Paulina Kettel says: “Our results reveal that IDRs provide the flexibility needed for clustering and initial assembly on membranes, where movement is restricted. This flexibility allows IRE1 to organize into oligomeric states, such as fibrillar structures, during stress, facilitating its activation.” Upon ER stress, disordered regions of multiple IRE1 molecules associate, with molecular crowding on the membrane surface driving regulated self-assembly. Elif Karagöz adds: “Our data show that different IDRs in the protein either promote or inhibit clustering, acting as molecular switches that tune IRE1’s self-association.”
The researchers’ discovery that previously overlooked regions of IRE1 play crucial regulatory roles could have significant implications for drug development. Targeting the organization of IRE1’s luminal domain with small molecules might offer a more specific approach compared to current drugs that target the highly conserved kinase domain. Such an approach could permit tailored therapeutic strategies, such as activating the UPR in cancer or inhibiting it in neurodegenerative diseases.
See the Max Perutz Labs News here.
Publication:
Disordered regions in the IRE1α ER lumenal domain mediate its stress-induced clustering.
EMBO Journal. 2024 September 4.
DOI: 10.1038/s44318-024-00207-0
Authors:
Paulina Kettel, Laura Marosits, Elena Spinetti, Michael Rechberger, Caterina Giannini, Philipp Radler, Isabell Niedermoser, Irmgard Fischer, Gijs A Versteeg, Martin Loose, Roberto Covino, G Elif Karagöz.
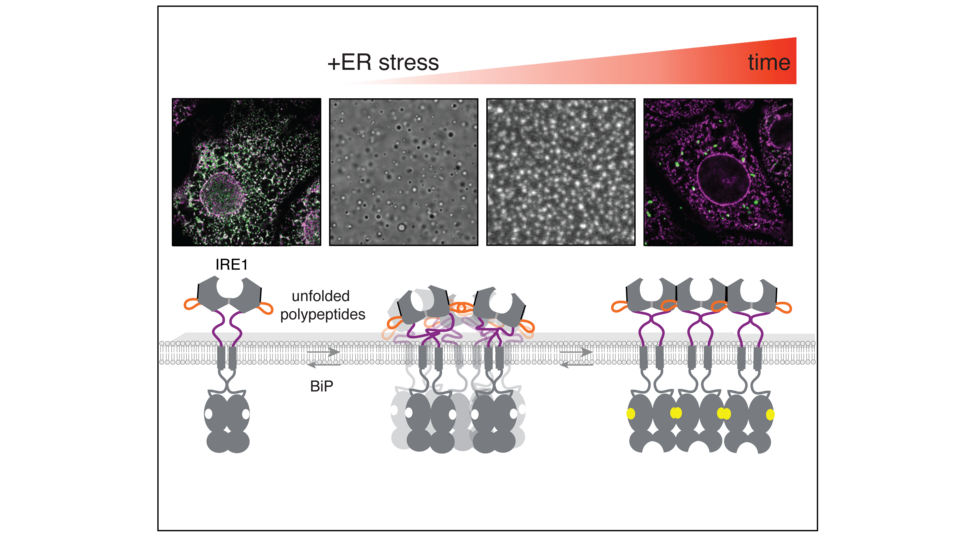
The figure shows IRE1 clusters observed in cells and in vitro reconstitution assays. The images represent different assembly intermediates of IRE1 and the importance of the disordered segments in IRE1 self-assembly. © Elif Karagöz.

Max Perutz Labs group leader Elif Karagöz (left) together with first author Paulina Kettel (right). © Max Perutz Labs.