October 24, 2024
Controlling condensation by phosphorylation
Condensate formation as an organizing principle is rapidly gaining traction in many biological processes. However, the mechanisms by which the size, composition and subcellular localization of these condensates are regulated are largely unknown. Harald Hornegger, a PhD student in the Karagöz lab at the Max Perutz Labs, has revealed that phosphorylation of the RNA-binding protein IGF2BP1 significantly impacts the biogenesis, size and number of stress granules. The study, published in Nature Communications, shows that post-translational modification of intrinsically disordered regions of IGF2BP1 alters its condensation properties, a mechanism which the authors propose allows cells to tune their transcriptional output in response to specific environmental conditions.
See the Max Perutz Labs News here.
Historically, compartmentalization of biological processes within eukaryotic cells has been framed in the context of membrane-bound organelles. However, it has long been known that specific groups of macromolecules, including both proteins and nucleic acids, can associate within the cell in so-called membrane-less organelles. “Cells contain specialized compartments known as condensates, which form without membranes through the self-association of molecules with similar physical properties”, Harald Hornegger, PhD student in the lab of Elif Karagöz and first author of the study, explains. “These condensates play a crucial role in organizing the crowded cellular environment by compartmentalizing specific biochemical processes into distinct regions within the cell.”
During cellular stress, the RNA-binding protein IGF2BP1, along with its target RNAs, is sequestered in ribonucleoprotein stress granules where, it is believed, the RNAs are protected from degradation until translation resumes. However, the regulatory mechanisms governing the condensation of IGF2BP1 have not been elucidated. The Karagöz lab has discovered that the phosphorylation of intrinsically disordered regions (IDRs) in IGF2BP1 significantly influences its condensation properties. “We found that phosphorylation modifies low-specificity interactions between proteins and RNA within a highly concentrated environment. These alterations can either strengthen or weaken the granules, thereby impacting the overall RNA levels in the cells during stress”, explains Harald.
The researchers’ findings reveal that phosphorylation of one IDR does not alter the protein’s intrinsic ability to bind RNA, but affects the affinity of binding, leading to smaller granules and an altered stress response. Group leader Elif Karagöz notes: “What was particularly intriguing was the effect of a single point mutation in the IDR of IGF2BP1 on the transcriptome of cells. When we introduced a phospho-mimetic mutant to replicate the biophysical properties of phosphorylation, we observed dramatic changes in the total transcriptome compared to the wild-type protein.
IGF2BP1 is typically upregulated during development and embryogenesis, but significantly downregulated in adult cells. However, studies have correlated over-expression of IGF2BP1 with highly aggressive tumors, making it a focus of research into therapeutic targets in cancer. Elif explains: “Cancer cells, due to their high metabolic demands, aneuploidy, and inherent proteotoxic stress, exist in a constant state of stress. Our study revealed that, under oxidative stress, IGF2BP1 phosphorylation is upregulated, whereas, during ER stress, phosphorylation is downregulated.” The scientists’ findings suggest that cells can fine-tune the availability of IGF2BP1 and its associated RNAs according to the type of stress they encounter.
See the Max Perutz Labs News here.
Publication:
IGF2BP1 phosphorylation in the disordered linkers regulates ribonucleoprotein condensate formation and RNA metabolism.
Nature Communications. 2024 October 20.
DOI: 10.1038/s41467-024-53400-4
Authors:
Harald Hornegger, Aleksandra S Anisimova, Adnan Muratovic, Benjamin Bourgeois, Elena Spinetti, Isabell Niedermoser, Roberto Covino, Tobias Madl, G Elif Karagöz.

Group leader Elif Karagöz together with first author Harald Hornegger. © Max Perutz Labs.
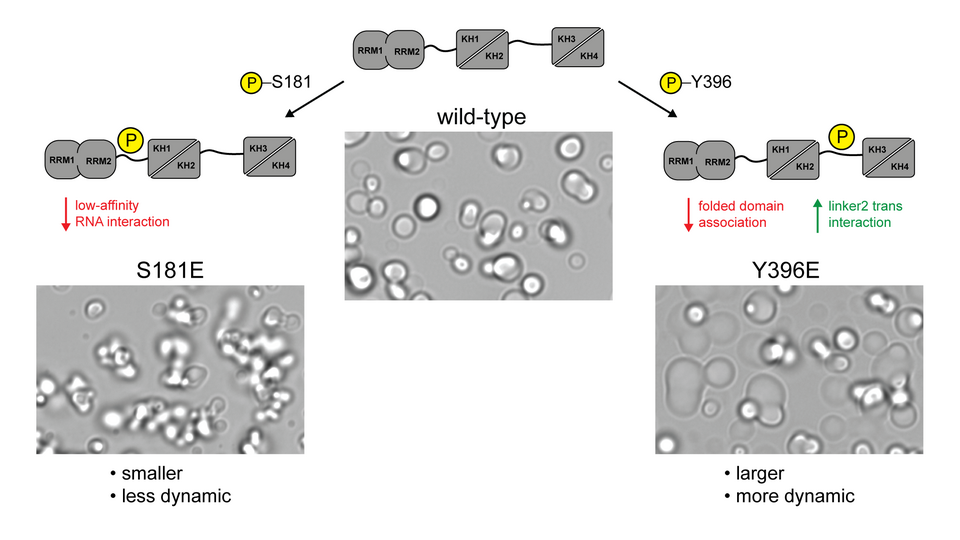
Phosphorylation in disordered linkers regulates properties of IGF2BP1 ribonucleoprotein condensates. © Harald Hornegger.