Group Urbán
Effects and regulation of proteostasis in the activation of adult stem cells
Group Leader
Noelia Urbán
Noelia was trained as a developmental neurobiologist in Barcelona. During her postdoc in London her focus shifted towards adult hippocampal neurogenesis. Currently at IMBA in Vienna, her group investigates how adult neural stem cells respond to systemic stimuli.
- Institute IMBA - Institute of Molecular Biotechnology
- Phone +43 1 79044 4920
- Mail noelia.urban@imba.oeaw.ac.at
- Web https://www.imba.oeaw.ac.at/research/noelia-urban/
Projects within consortium
Controlled protein degradation is critical during cellular transitions, like differentiation or exit from quiescence. When cells change their state, they must ensure not only that new gene products are generated but also that old cellular determinants that were locking their previous state are efficiently eliminated. We investigate the role and regulation of proteostasis in the transition of adult hippocampal stem cells from quiescence to activation.
In the hippocampus, adult neural stem cells (aNSCs) generate new neurons that modulate memory, stress and anxiety. Most aNSCs are in a resting state called quiescence, and their recruitment into the cell cycle is the most important regulatory step in adult neurogenesis. Systemic stimuli, such as exercise, diet or stress have a great impact on adult neurogenesis, but little is known about how aNSC quiescence is regulated by these stimuli.
We previously found that the E3-ubiquitin ligase Huwe1 is essential to allow the return to quiescence of activated aNSCs but has little effect on quiescent aNSCs. This has led us to hypothesize that quiescent and active stem cells might use different strategies for protein degradation. Indeed, proteostasis is a major regulator of aNSCs, which switch from using mostly the autophagosome in quiescence to rely on the ubiquitin-proteasome system (UPS) when proliferating. We aim to find the links between systemic signals, proteostasis and the regulation of aNSC quiescence.
To tackle this question, we have generated an in vitro system that recapitulates the preferential use of autophagy in quiescent and of the UPS in proliferating aNSCs. This gives us a unique opportunity to explore the mechanisms by which proteins are targeted to one or the other system for degradation and to understand how these general processes regulate neurogenesis.
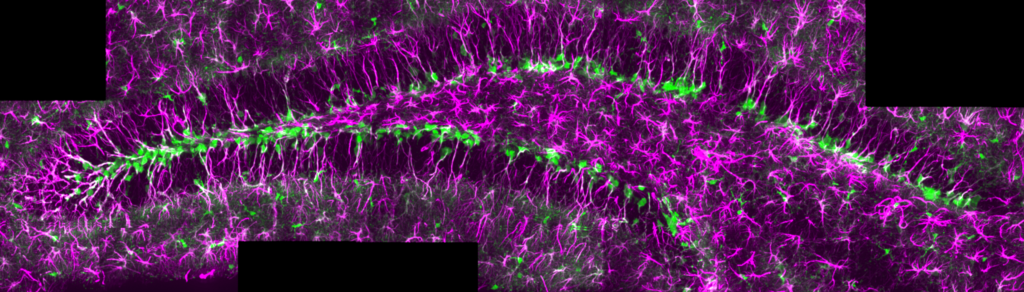
Adult neural stem cells in the adult hippocampus of a GlastCREert2/RYFP mouse. GFAP (magenta), recombination of the RYFP cassette (green).
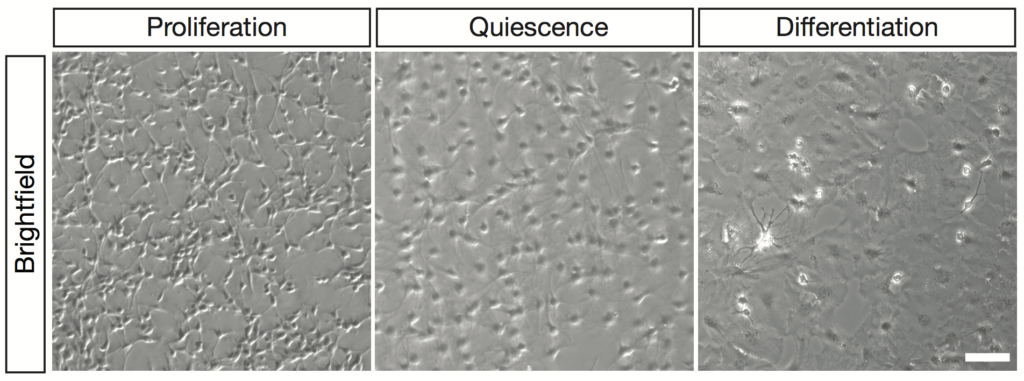
Adult hippocampal stem cells in vitro can acquire a quiescence state and differentiate into astrocytes and neurons.
Project members
-
Postdoc
Ana Paula Zen Petisco Fiore
SFB Member
-
PhD Student
Greeshma Pushpa Bose
Associated
-
Senior Research Assistant
Lilian Fennell
former SFB Member (until 12-2024)
Targeted Protein Degradation related publications by Group Urbán
- 2023 Adult neural stem cells and neurogenesis are resilient to intermittent fasting EMBO Journal Go to publication →
- 2022 Could a Different View of Quiescence Help Us Understand How Neurogenesis Is Regulated? Frontiers in Neuroscience Go to publication →
- 2021 Stem cell quiescence: the challenging path to activation Development Go to publication →
- 2021 Wnt/beta-catenin signalling is dispensable for adult neural stem cell homeostasis and activation Development Go to publication →