December 4, 2024
Seipin: Guardian of Lipid Balance
The inner nuclear membrane, a hub of genome regulation and nuclear envelope organization, holds untapped mysteries in lipid metabolism. In a study published in Nature Communications, the Köhler lab from the Max Perutz Labs identified Seipin – a protein previously linked to a severe lipid storage disorder – as a key regulator of inner nuclear membrane lipid homeostasis and nuclear envelope integrity.
The nuclear envelope is a dual-membrane structure that separates the genome from the rest of the cell. The outer nuclear membrane is continuous with the endoplasmic reticulum, while the inner nuclear membrane (INM) is a specialized domain essential for regulating gene expression. Once considered metabolically inert, the INM was revealed by the Köhler group in 2018 to exhibit active lipid metabolism. In this new study, a genome-wide lipid biosensor screen targeting phosphatidic acid – a precursor for both phospholipids and storage lipids – identified Seipin as a critical regulator of of inner nuclear membrane lipid homeostasis.
Seipin has traditionally been recognized for its role in lipid droplet formation in the cytoplasm, where it assembles into a ring-like structure resembling a valve (see image) to mediate the controlled transfer of triacylglycerol from membranes into lipid droplets. Unexpectedly, a lipid biosensor screen revealed a novel function for Seipin in maintaining phosphatidic acid homeostasis at the INM, thereby preventing structural defects in the nuclear envelope. Beyond its role in phosphatidic acid regulation, Seipin also governs lipid droplet formation within the nucleus – a process that becomes defective when Seipin is absent or dysfunctional. Anete Romanauska, first author of the study, says: “Building on these findings, we identified 10 additional Seipin-associated regulators of nuclear lipid droplet formation and demonstrated Seipin’s impact on distinct lipid species of the INM.“ Using AlphaFold-driven modeling and mutational analyses, they further dissected the Seipin complex, showing that the regulation of phosphatidic acid and the channeling of triacylglycerol into nuclear lipid droplets are mediated by separate regions of the complex. This functional partitioning highlights the intricate regulation of lipid species within the nuclear membrane.
“This study advances our understanding of nuclear lipid dynamics and highlights genome-wide lipid biosensor screens – developed in collaboration with Maya Schuldiner at the Weizmann Institute – as a powerful tool for investigating inner nuclear membrane lipids”, adds group leader Alwin Köhler. The identification of Seipin as a ‘Guardian of Lipid Balance’ at the INM paves the way for exploring connections between nuclear lipid dysregulation and Berardinelli-Seip congenital lipodystrophy, a severe lipid storage disorder.
See the Max Perutz Labs News here.
Publication:
Seipin governs phosphatidic acid homeostasis at the inner nuclear membrane.
Nature Communications. 2024 Dec 2.
DOI: 10.1038/s41467-024-54811-z
Authors:
Read more about the Köhler lab in our Special Research Program and at the Max Perutz Labs.
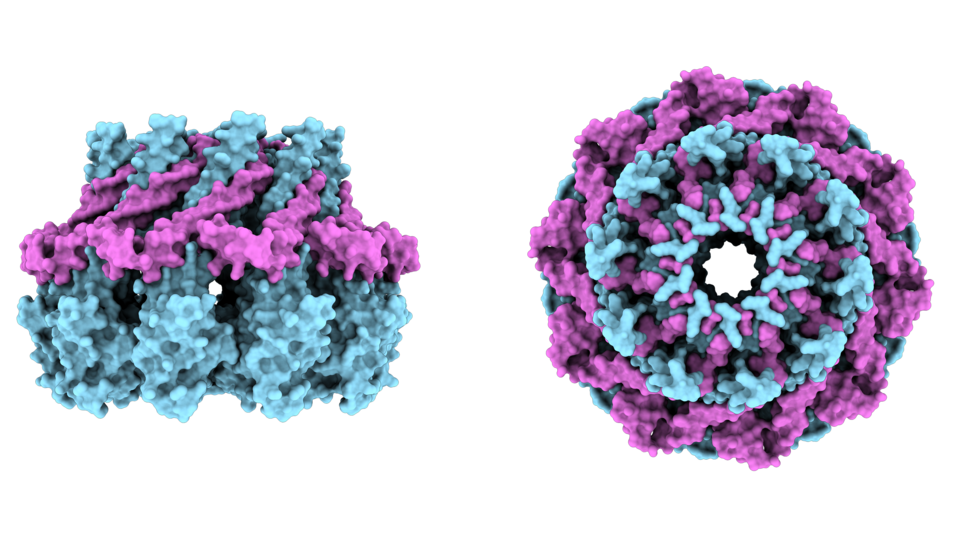
Seipin is a membrane protein that assembles into a ring-like structure, similar to a valve. This configuration facilitates the controlled channeling of triacylglycerol from membranes into lipid droplets. An AlphaFold3 model of the Sei1-Ldb16 ring assembly (cyan and magenta, respectively) provides views from the side (left) and into the valve-like interior (right). © Alwin Köhler