October 25, 2024
Time for lunch
Aggregated, misfolded proteins need to be removed by cells to avoid their build-up. Failure to do so often leads to disease. During a process called aggrephagy, aggregated proteins are marked with ubiquitin and clustered into molecular condensates, which are then degraded with the help of the autophagic machinery. In a new study published in EMBO Journal, Bernd Bauer from the the Martens group (Max Perutz Labs) reveals how the cargo receptor protein TAX1BP1 facilitates the switch from cargo collection to autophagosome formation. Bernd’s findings show that TAX1BP1 recruits pro-autophagic factors to initiate autophagosome biogenesis by sensing the amount of ubiquitin within the cargo.
See the Max Perutz Labs News here.
Cells dispose of waste through autophagy, a process in which material is encapsulated in a double membrane, called the autophagosome, which targets it to the lysosome for degradation and recycling. To degrade unfolded or misfolded proteins, a specialized form of autophagy known as aggrephagy is required: protein aggregates marked with ubiquitin are recognized and clustered by the ubiquitin-binding protein p62 before being encapsulated by the autophagosomal membrane. The protein TAX1BP1 is known to be required for the initiation of autophagosome formation, but the mechanism by which it drives the switch from cargo condensation to autophagosome formation is unknown.
TAX1BP1 is not a constitutive component of the condensates but plays a crucial role in driving autophagosome biogenesis. First author Bernd Bauer explains: “We discovered that ubiquitin, often acting as the cell’s ‘eat me signal’ for degradation, is an important factor in recruiting TAX1BP1. This recruitment, as we now understand, triggers the initiation of autophagy at these condensates.” Bernd adds: “Interestingly, TAX1BP1 competes with ATG8-like proteins, which canonically decorate the autophagosomal membrane, for binding to another cargo receptor NBR1. This suggests a two-step recruitment process, in which ubiquitin helps TAX1BP1 overcome this competition, allowing it to bind NBR1 and stably initiate autophagy by recruiting upstream signaling components.” Following this, the cell transitions to condensate encapsulation, ultimately leading to cargo degradation.
Pathological protein aggregation plays a key role in a number of neurodegenerative diseases. The Martens lab previously revealed how protein aggregates in Alzheimer’s patients evade binding by TAX1BP1, thereby escaping aggrephagy. The new study adds an important mechanistic insight into how TAX1BP1 drives membrane encapsulation of aggregates, which will help researchers in the future to understand how some aggregates can escape the cell’s waste disposal machinery.
Publication:
Recruitment of autophagy initiator TAX1BP1 advances aggrephagy from cargo collection to sequestration.
EMBO Journal. 2024 October 24.
DOI: 10.1038/s44318-024-00280-5
Authors:
Bernd Bauer, Jonas Idinger, Martina Schuschnig, Luca Ferrari, and Sascha Martens.
See the Max Perutz Labs News here.
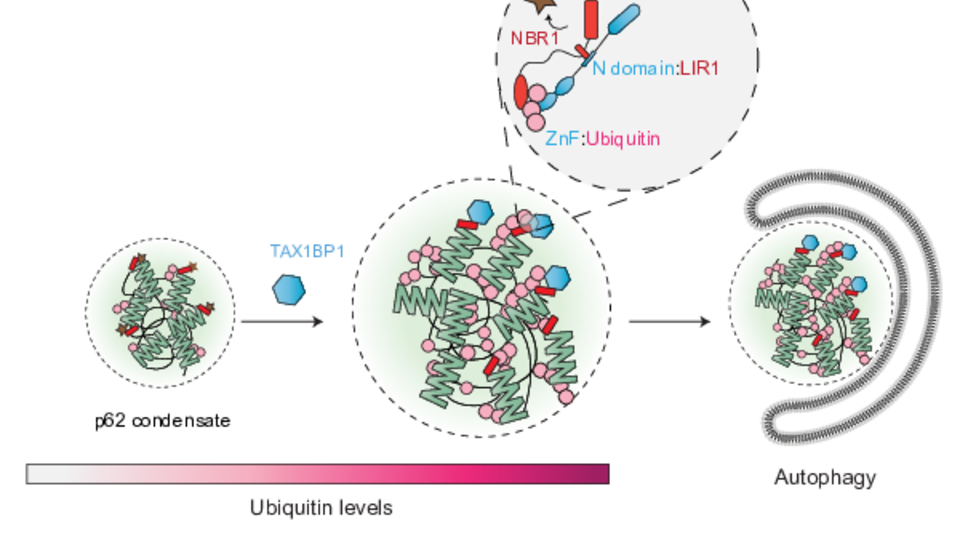
In aggrephagy, ubiquitinated and unfolded proteins are collected in condensates prior to their sequestration in to autophagosomes; however, the transition between these two processes were elusive. The researchers found that the recruitment of the autophagy initiator TAX1BP1 can be triggered by increased ubiquitin levels in these condensates. © Bernd Bauer.

Group leader Sascha Martens (left) and first author Bernd Bauer (right). © Max Perutz Labs.