June 14, 2024
Unrecyclable: how protein aggregates evade clearance
Protein aggregates are a common hallmark of neurodegenerative diseases. These aggregates accumulate despite dedicated cellular surveillance mechanisms to prevent the build-up of unfolded or damaged cellular components. In a study published in Science Advances, Luca Ferrari, a postdoctoral researcher and member of our SFB in the Martens group at the Max Perutz Labs, compared how monomeric and pathological Tau proteins are targeted by this surveillance machinery. The team found that while Tau monomers are degraded normally, Tau fibrils, a hallmark of Alzheimer’s Disease, evade clearance by preventing the binding of a crucial mediator, TAX1BP1, which helps to recruit the autophagy machinery.
Tau proteins are small cytosolic proteins known for their ability to bind tubulin and regulate the cytoskeleton of cells. Under healthy conditions, Tau functions as a monomer, whereas under pathogenic conditions, Tau oligomerizes to form fibrils. The accumulation of Tau fibrils is observed in the brains of patients suffering from Alzheimer’s disease. First author Luca Ferrari explains: “In Alzheimer’s research, we do not know if the accumulation of these aggregates is what causes neurodegeneration. However, we always see massive aggregates of Tau protein together with neurodegeneration.”
Luca explains further: “The scientific question is why Tau accumulates in the brain, because normally the brain has ways to remove trash.” Autophagy is a process resembling a city’s waste management. Ubiquitinated proteins, so called cargoes, are the signal for autophagy receptors to recruit the autophagy machinery and mediate degradation of the protein in the lysosome. “For effective clearance, you need a series of autophagy receptors to assemble sequentially”, Luca emphasizes. The scientists’ results show that ubiquitinated Tau monomers are recognized first by p62, followed by NBR1, which in turn recruits TAX1BP1.
By contrast, although Tau fibrils isolated from Alzheimer’s disease affected brains also show ubiquitination followed by the binding of p62 and NBR1, the fibrils fail to recruit TAX1BP1. “In our study, we show that there is not enough ubiquitin to recruit TAX1BP1”, Luca says. The failure to recruit TAX1BP1 leads to a failure in autophagosome formation, and therefore Tau fibrils accumulate in brain cells, the team hypothesizes.
The approach of the Martens lab is unique: their collaboration with the Medical University of Vienna allows them to investigate healthy and diseased post-mortem brain samples instead of working with artificial substrates. After the purification of the aggregates, the biochemical reconstitution expertise from the Martens lab comes in. Luca emphasizes: “This is the perfect approach to understand the mechanisms behind the disease.
And there is more: neurodegenerative diseases in general are characterized by protein aggregates. The pathological proteins themselves differ from disease to disease, but our approach could be applicable to other diseases.” Not only does the approach provide new insights into Alzheimer’s, but the study itself is also of clinical relevance. Pharmacological tethering of TAX1BP1 to Tau fibrils, if possible, may rescue the deficiency in autophagy – a novel, and potentially promising, approach in the fight against neurodegenerative diseases.
Publication:
Tau fibrils evade autophagy by excessive p62 coating and TAX1BP1 exclusion.
Science Advances. 2024 June 12.
Authors:
Luca Ferrari, Bernd Bauer, Yue Qiu, Martina Schuschnig, Sigrid Klotz, Dorothea Anrather, Thomas Juretschke, Petra Beli, Ellen Gelpi, Sascha Martens.
See the Max Perutz Labs News here.

First author Luca Ferrari (left) and group leader Sascha Martens (right). © Max Perutz Labs.
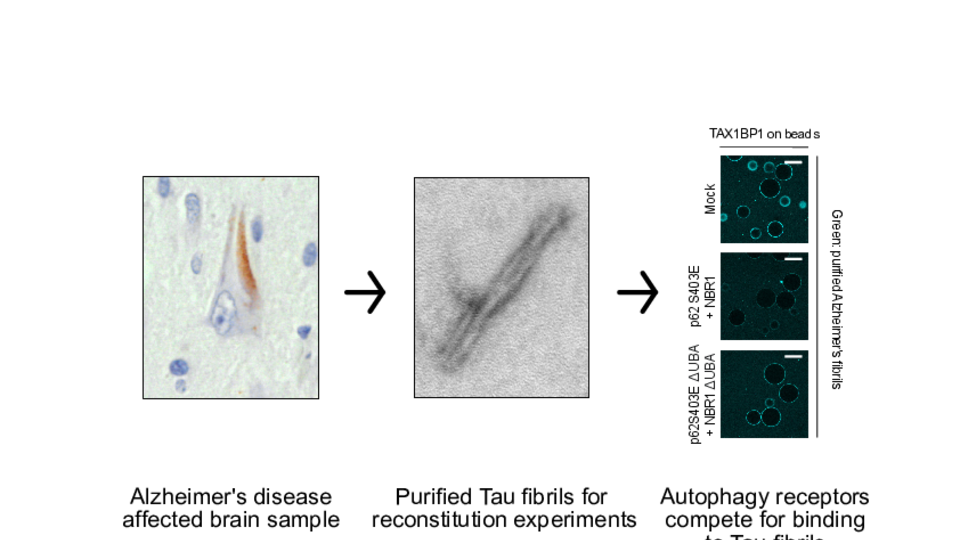
The three autophagy receptors p62, NBR1 and TAX1BP1 compete for the binding of to Tau fibrils. TAX1BP1 is outcompeted, explaining fibrils accumulation in disease. © Luca Ferrari.